Ask & Answer: India's Largest Education Community
All Questions
New Question
4 months agoContributor-Level 10
Candidates can check the JEECUP answer key through their candidate login portal using their application number and password.
New Question
4 months agoContributor-Level 10
No, it doesn't change. The National Testing Agency (NTA) has been consistently responsible for conducting the Common University Entrance Test (CUET) PG since its inception.
New Question
4 months agoContributor-Level 9
In thin layer chromatography, the spots of colourless compounds, which are invisible to eyes can be detected by putting the plate under UV light.
Another detection technique is to place the plate in a covered jar containing I2 (s). Sometimes an appropriate reagent may also be sprayed on the plate, ninhydrin in case of amino acids.
New Question
4 months agoContributor-Level 10
Undergraduate courses offered at IHMs are as follows:
BSc in Hospitality and Hotel Administration (HHA)
BA in Culinary Arts
BSc in IT
BSc Computer Science
Although there are many other courses taught in IHMs India, however, these courses are very popular among Central IHMs, State IHMs, and Private IHMs.
New Question
4 months agoContributor-Level 10
There is a total of 71 Institutes of Hotel Management that is IHMs in India. The number includes both Central Government-sponsored IHMs and State Government-sponsored IHMs.
New Question
4 months agoContributor-Level 10
The JEECUP result will comprise the candidate's name, roll number, marks obtained and the rank secured in the exam.
New Question
4 months agoContributor-Level 9
CN- is strong field ligand
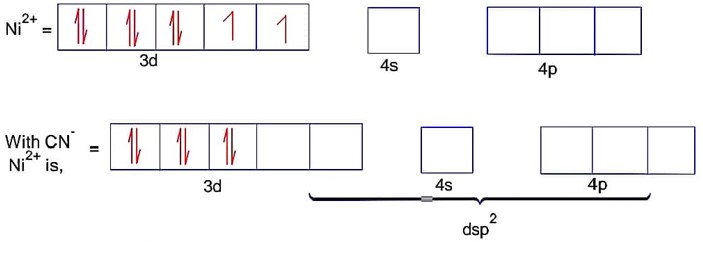
Here; is square planar and diamagnetic.
Ni = 4s23d8 Co is strong field ligand.
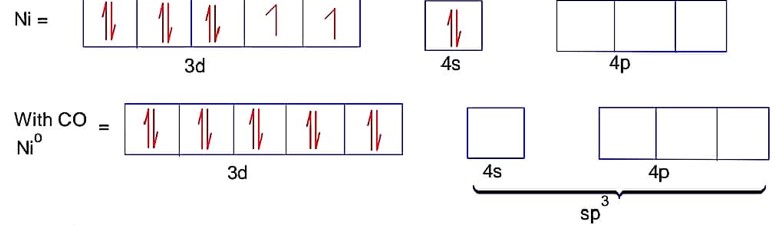
Here ; is tetrahedral and diamagnetic
has 3d8 configuration while has 3d10 configuration.
New Question
4 months agoContributor-Level 10
The JEECUP result link will be updated on the official website, jeecup.admissions.nic.in. Candidates can check their JEECUP scores through the candidate login portal using their application number and password.
New Question
4 months agoContributor-Level 10
The authority will announce the JEECUP 2026 result date online along with the exam notification.
Register to get relevant
Questions & Discussions on your feed
Ask & Answer
Panel of Experts