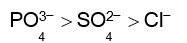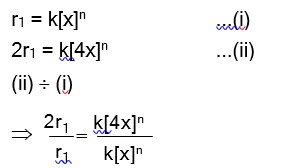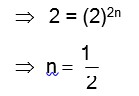Ask & Answer: India's Largest Education Community
All Questions
New Question
4 months agoContributor-Level 10
There are two types of electrochemical cells - Electrolytic and Galvanic or Voltaic cells. The electrolytic cells need an external source such as AC power source or DC battery and it involve non-spontaneous reactions. The galvanic cells gets its energy from redox reactions which is spontaneous.
New Question
4 months agoContributor-Level 10
Redox reactions is the basic principle of the electrochemistry. The redox reactions is the process where electrons are transferred between substances. In this process chemical energy gets converted into electrical energy and vice versa.
New Question
4 months agoContributor-Level 10
[Co (NH3)6]3+, NH3 becomes strong ligand due to +3 oxidation state of cobalt ion. Hence electronic configuration of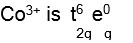
New Question
4 months agoContributor-Level 10
In simple words, the study of relationship between electrical energy and chemical reactions is called the Electrochemistry. The concept comprises how chemical reactions can create electrical energy and how electrical energy can generate chemical changes.
New Question
4 months agoContributor-Level 10
The past year's NSI Admission Test have some important things to think about.
Getting to Know the Exam Format. Understanding the structure of the test and the types of questions and how difficult they are to answer is critical for test success. Students are often surprised and ill-prepared for how challenging and exhaustive the tests are. Knowing the level of difficulty beforehand can change and improve the outcomes of prospective candidates.
New Question
4 months agoContributor-Level 6
The last date to fill the JKLU EET 2025 application form for Round 2 was April 2025.
New Question
4 months agoContributor-Level 7
The application fee for General, 2A, 2B, 3A and 3B who wants to apply for Paper I and II is INR 1000. It is INR 700 for either Paper 1 or 2.
New Question
4 months agoNew Question
4 months agoContributor-Level 6
Candidates meeting the eligibility criteria can only fill the application form.
New Question
4 months agoContributor-Level 10
Haemoglobin is a positive colloid. Hence greater is the charge of anion, more effective will be the coagulation of haemoglobin.
Therefore,
Correct order of coagulating power is
New Question
4 months agoContributor-Level 7
KARTET application form can be filled online only. The steps to fill the application form are given below.
- Visit the official website
- Click the apply online link
- Select the paper for which candidates want to submit the application form
- Fill the required details
- Pay the fees
- Submit the online form
New Question
4 months agoContributor-Level 6
The application form is available in online mode. The registeration form is available on the official website.
New Question
4 months agoContributor-Level 10
Electrophoresis : The migration of colloidal particles under the influence of an electric field is known as electrophoresis.
New Question
4 months agoContributor-Level 7
The basic educational qualification required for KARTET exam is Graduate with B.Ed. The educational qualification vary as per posts.
New Question
4 months agoContributor-Level 7
The minimum Karnataka TET age limit is 18 years. There is no limit in the number of attempts. The eligibility certificate for KARTET is valid for lifetime.
Register to get relevant
Questions & Discussions on your feed
Ask & Answer
Panel of Experts