Match List I with List II.
List I List II
(a) Isothermal (i) Pressure constant
(b) Isochoric (ii) Temperature constant
(c) Adiabatic (iii) Volume constant
(d) Isobaric (iv) Heat content is constant
Choose the correct answer from the options given below:
Match List I with List II.
List I List II
(a) Isothermal (i) Pressure constant
(b) Isochoric (ii) Temperature constant
(c) Adiabatic (iii) Volume constant
(d) Isobaric (iv) Heat content is constant
Choose the correct answer from the options given below:
In isothermal process, temperature is constant.
In isochoric process, volume is constant.
In adiabatic process, there is no exchange of heat.
In isobaric process, pressure is constant
Similar Questions for you
Heat lost by steam Heat gained by water and calorimeter.
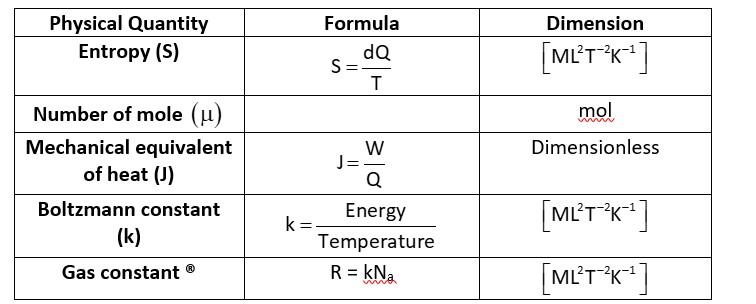
Na -> Avogadro’s Number
, and
-> Dimensionless
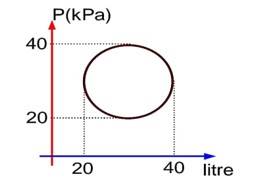
Heat absorbed in cyclic process = Work done = 100? Joule
Since process is isochoric, so
Since process is isochoric
So
And external work
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...
Didn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering