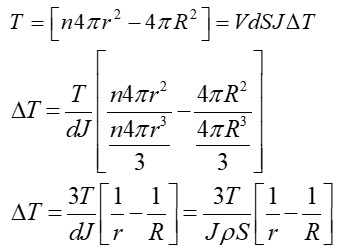n droplets of water each of radius r coalesce to form a big drop of radius R. If the energy released during coalescence goes into heating the drop, then the rise in temperature will be (take T as surface tension of water and J as mechanical equivalent of heat)
n droplets of water each of radius r coalesce to form a big drop of radius R. If the energy released during coalescence goes into heating the drop, then the rise in temperature will be (take T as surface tension of water and J as mechanical equivalent of heat)
Similar Questions for you
Gain in surface energy,
from volume centenary,
Initial surface area, Ai = 4pR2
final surface area,
Fact. Due to decrease in intermolecular forces
P? - P? = 4T/a
P? - P? = 4T/b
P? - P? = 4T (1/a - 1/b)
Also, P? - P? = 4T/r
4T/r = 4T (1/a - 1/b)
1/r = (b-a)/ab
r = ab / (b-a)
From volume conservation
Decrease in surface area =
Energy released (W) =
Heat produced (Q) =
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Physics Mechanical Properties of Fluids 2025
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering