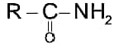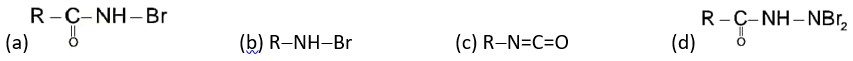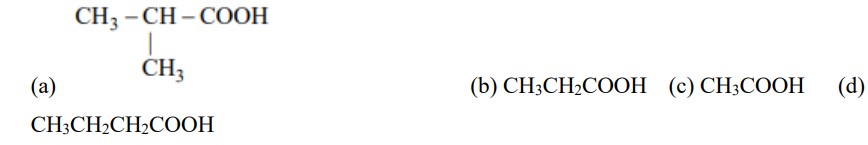Ask & Answer: India's Largest Education Community
All Questions
New Question
5 months agoNew Question
5 months agoContributor-Level 10
Kf = 1.86
Using, density of water = 1g / mL
i = ?
i = 1.344
Now, using
n for ClCH2COOH = 2
a =
Using
So; x = 36
New Question
5 months agoContributor-Level 10
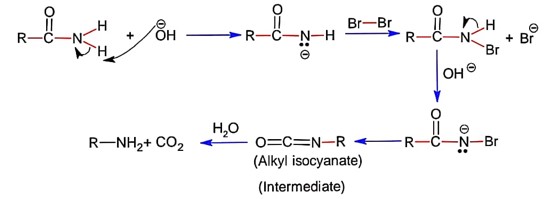
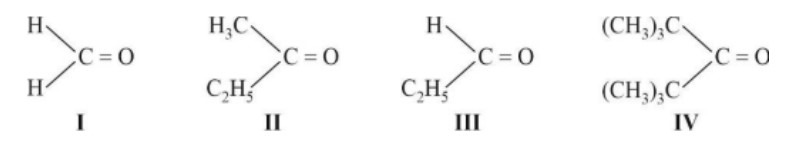
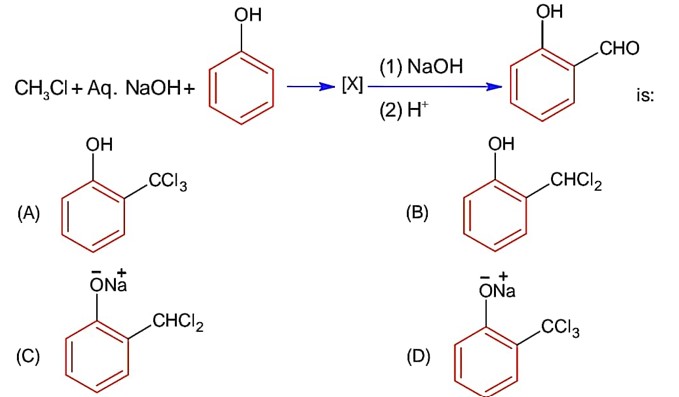
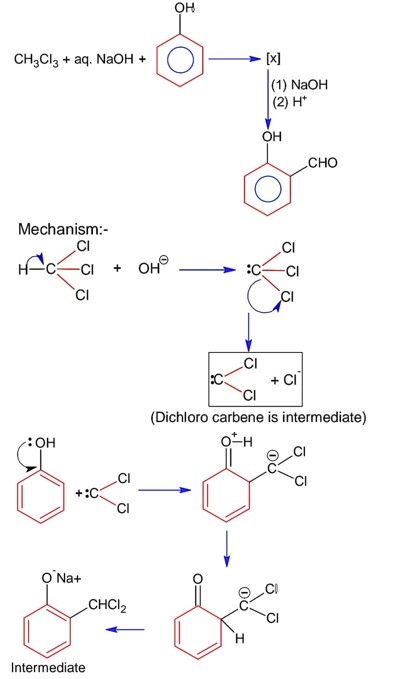
If the carbonyl compound is sterically crowded, then it will be reluctant to undergo addition reaction. Moreover, attachment of bulkier alkyl group with the carbonyl carbon decreases the partial positive charge resulting into the minimization of attack by R? from RMgBr. So, the order is
New Question
5 months agoContributor-Level 9
According to Gauss’s law for magnetism, the net magnetic flux through any closed surface is zero.
It states that all magnetic field lines must enter and exit from a surface. In this way, it differs from electric charges, which can exist independently as positive and negative.
New Question
5 months agoContributor-Level 9
Magnetism is the force exerted by a magnet. It comes when the magnet attracts or repulses an object. For example, a magnet sticks to certain objects because the magnetic force or magnetism pulls it.
New Question
5 months agoContributor-Level 10
Initially -> 1 mol -
At eq. 1-x mol 2x mol
Here; molecules of Cl2 = atoms of Cl
i.e moles of Cl2 = moles of Cl
So : 1 – x = 2x x = 1/3
Moles of Cl2 at
New Question
5 months agoContributor-Level 10
A (g) -> B (g) KP = 100
at 300 K and 1 atm
Using
=-R × 300 × 2 × 2.3
So; x = 1380.
New Question
5 months agoContributor-Level 10
Stability constant are :
K1 = 104
K2 = 1.58 × 103
K3 = 5 × 102
K4 = 102
Overall stability constant K will be
K = K1 × K2 × K3 × K4
= 7.9 × 1011
Now, overall equilibrium constant for dissociation of [Cu (NH3)4]2+ is
= 1.26 × 10-12
So; x = 1 (Rounded off to the nearest integer)
New Question
5 months agoContributor-Level 10
Beo & Be (OH)2 are amphoteric in nature, because they react with both acid and base
New Question
5 months agoContributor-Level 10
The Jadavpur University is ranked 18 by the NIRF in 2025. However, the NIRF rank for other similar institutes is around 20 - 45 ranks which means that the BE courses at Jadavpur University follow a higher level of quality for their course.
New Question
5 months agoContributor-Level 10
Methane gas is produced during anaerobic degradation of vegetation that leads to global warming so considered as greenhouse gas like CO2. Also it causes cancer.
New Question
5 months agoContributor-Level 9
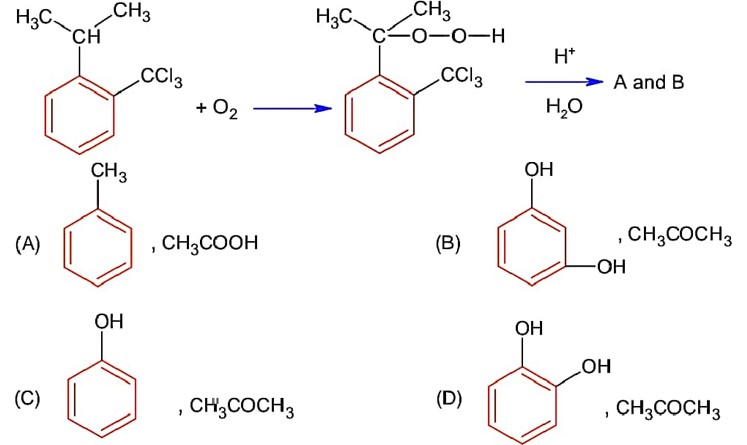
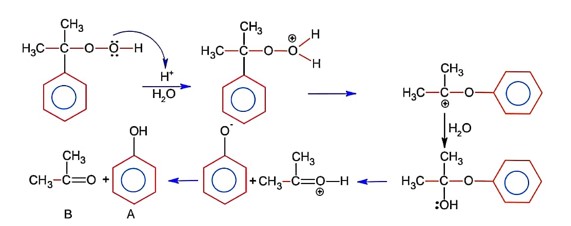
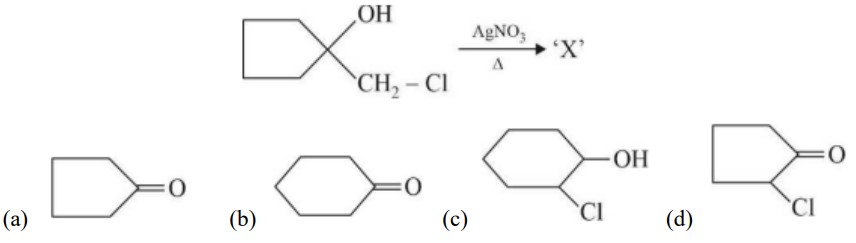
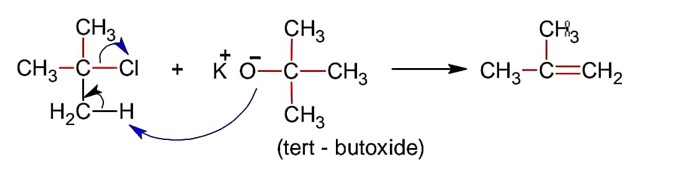
Base is tert-butoxide thus favours elimination with 3° alkyl halide and gives alkene
New Question
5 months agoContributor-Level 10
Andhra University provides good packages to its graduating students.However, the institution has not released the course wise placement report as of yet. In the meantime, check out the table below to know the median package recorded during Andhra University placements 2024:
Particulars | Placement Statistics (2024) |
|---|---|
Median Package | INR 4.10 LPA (UG 3-year) INR 7.58 LPA (UG 4-year) INR 3.90 LPA (UG 5-year) INR 6.70 LPA (PG 2-year) INR 4.50 LPA (PG 3-year) INR 4.50 LPA (PG 6-year) |
New Question
5 months agoContributor-Level 10
The institution has not released the course-wise placement packages as of yet. Meanwhile the NIRF report has been released. Check out the table below to know more:
Particulars | Placement Statistics (2022) | Placement Statistics (2023) | Placement Statistics (2024) |
|---|---|---|---|
Total Students | 75 (UG 3-year) 449 (UG 4-year) 101 (UG 5-year) 2,901 (PG 2-year) 9 (PG 3-year) 29 (PG 6-year) | 36 (UG 3-year) 746 (UG 4-year) 104 (UG 5-year) 2,959 (PG 2-year) 11 (PG 3-year) 30 (PG 6-year) | 40 (UG 3-year) 761 (UG 4-year) 100 (UG 5-year) 2,432 (PG 2-year) 11 (PG 3-year) 30 (PG 6-year) |
No. of Students Placed | 42 (UG 3-year) 415 (UG 4-year) 86 (UG 5-year) 2,345 (PG 2-year) 6 (PG 3-year) 17 (PG 6-year) | 22 (UG 3-year) 652 (UG 4-year) 90 (UG 5-year) 2,499 (PG 2-year) 10 (PG 3-year) 25 (PG 6-year) | 24 (UG 3-year) 646 (UG 4-year) 90 (UG 5-year) 2,067 (PG 2-year) 10 (PG 3-year) 26 (PG 6-year) |
Median Salary | INR 3 LPA (UG 3-year) INR 7.50 LPA (UG 4-year) INR 2.75 LPA (UG 5-year) INR 6.50 LPA (PG 2-year) INR 3.50 LPA (PG 3-year) INR 3 LPA (PG 6-year) | INR 3.55 LPA (UG 3-year) INR 7.52 LPA (UG 4-year) INR 3.90 LPA (UG 5-year) INR 6.62 LPA (PG 2-year) INR 4.50 LPA (PG 3-year) INR 4.50 LPA (PG 6-year) | INR 4.10 LPA (UG 3-year) INR 7.58 LPA (UG 4-year) INR 3.90 LPA (UG 5-year) INR 6.70 LPA (PG 2-year) INR 4.50 LPA (PG 3-year) INR 4.50 LPA (PG 6-year) |
NOTE: The above data is obtained from the NIRF report 2025
New Question
5 months agoContributor-Level 10
For stabilization of a - helix structure of protein, H-bonding is responsible which is in between 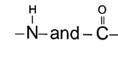
New Question
5 months agoContributor-Level 10
ICT Mumbai cutoff 2025 was concluded with the release of the final cutoff list for admission to the BTech and Integrated BTech courses. During the last round of the ICT Mumbai JEE Main cutoff 2025, the closing ranks varied between 22643 and 115110 for the students belonging to the General AI quota
Register to get relevant
Questions & Discussions on your feed
Ask & Answer
Panel of Experts