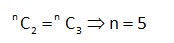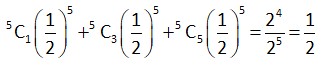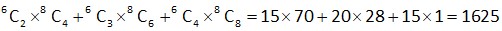Ask & Answer: India's Largest Education Community
All Questions
New Question
5 months agoContributor-Level 7
There has been no update on any changes in the JEE Advanced 2026 attempt limits. If any changes are to be made in the attempt limit, the same will be officially notified at jeeadv.ac.in. Currently, the JEE Advanced attempt limit is twice in two consecutive years.
New Question
5 months agoContributor-Level 10
Candidates can check the table below to know course-wise median package offered to UG 3-year, UG 4-year and PG 2-year:
Course | Median Package (2022) | Median Package (2023) | Median Package (2024) |
|---|---|---|---|
UG 3-year | INR 6 LPA | INR 8 LPA | INR 6.5 LPA |
UG 4-year | 0 | INR 10 LPA | INR 6.5 LPA |
PG 2-year | INR 7.95 LPA | INR 9.5 LPA | INR 9.5 LPA |
New Question
5 months agoContributor-Level 10
k = at ……… (ii)
From (i) & (ii)
Equation of directrix x – a/2 = -a/2Þ x = 0
New Question
5 months agoContributor-Level 7
IIT Roorke will start JEE Advanced registration 2026 on April 23, 2026 for Indian nationals. Application form will be available online on the website jeeadv.ac.in. The JEE Advanced application form 2026 for foreign nationals will be released on April 6, 2026
New Question
5 months agoContributor-Level 9
Fe+2 undergoes reduction & I2 undergoes oxidation.
= (0.77 – 0.54) V = 0.23 V
= 23 × 10-2 V
x = 23
![]()
New Question
5 months agoContributor-Level 10
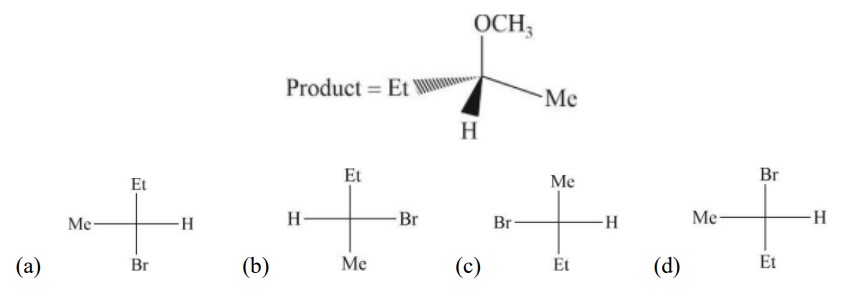
In S?2 reaction, inversion takes place, so configuration get reversed.
New Question
5 months agoContributor-Level 9
The placement trends witnessed during JNU placement over the past three years are presented below:
Particulars | UG 3-year Placement Statistics (2022) | UG 3-year Placement Statistics (2023) | UG 3-year Placement Statistics (2024) |
|---|---|---|---|
Median package | INR 6 LPA | INR 8 LPA | INR 6.5 LPA |
Total students | 353 | 350 | 357 |
Students placed | 21 | 25 | 30 |
Students selected for higher studies | 332 | 325 | 327 |
Particulars | UG 4-year Placement Statistics (2022) | UG 4-year Placement Statistics (2023) | UG 4-year Placement Statistics (2024) |
Median package | 0 | INR 10 LPA | INR 6.5 LPA |
Total students | 0 | 75 | 78 |
Students placed | 0 | 24 | 17 |
Students selected for higher studies | 0 | 6 | 61 |
Particulars | PG 2-year Placement Statistics (2022) | PG 2-year Placement Statistics (2023) | PG 2-year Placement Statistics (2024) |
Median package | INR 7.95 LPA | INR 9.5 LPA | INR 9.5 LPA |
Total students | 1280 | 1329 | 1432 |
Students placed | 86 | 12 | 188 |
Students selected for higher studies | 384 | 1317 | 1244 |
New Question
5 months agoContributor-Level 10
Jadavpur University has been ranked among the best institutes for BSc courses. While certain similar institutes such as BHU and Jamia Milia are ranked higher than Jadavpur, the difference between them is very minor. Other similar institutes are ranked much lower than Jadavpur Univeristy. The aforemention rank comparison was performed using the NIRF ranks of all institutes.
New Question
5 months agoContributor-Level 10
As Zn (OH)? , BeO, Al? O? are amphoteric, so they can react with both HCl and NaOH.
New Question
5 months agoContributor-Level 10
Let total number of throws = n
Probability of getting 2 times = Probability of getting an even number 3 times.
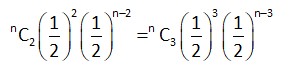
[as probability of getting odd number = probability of getting even number = ]
New Question
5 months agoContributor-Level 10
When Ferric chloride reacts with potassium thiocyanate a blood red colour of Ferric thiocyanate is formed
FeCl? + 3KSCN → Fe (SCN)? + 3KCl
New Question
5 months agoContributor-Level 7
The exam date for JEE Advanced 2026 has been announced. IIT Roorkee is the organising institute for JEE Advanced exam 2026. The exam will be held on May 17, 2026.
New Question
5 months agoContributor-Level 10
IIT Roorkee is the organising institute for JEE Advanced 2026 exam. Details related to the exam date, eligibility criteria and syllabus of JEE Advanced 2026 can be checked on the website jeeadv.ac.in.
New Question
5 months agoContributor-Level 10
Formation of passive layer of Fe? O? on the surface of Fe and NO? gas is evolved
[Co (NH? )? ]³? is diamagnetic (t? g? eg? ) and is inner orbital complex
New Question
5 months agoContributor-Level 9
1000 ml solution contains 0.02 milli mole (mm)
500 ml solution contains 0.02 m.m
Solution made 1000 ml with H2O
m.m in final solution = 0.01 mm
Solution (A) + 0.01 m. m H2SO4
= 0.01 + 0.01
= 0.02 m.m
= 0.00002 × 103 mm
New Question
5 months agoContributor-Level 10
i. Zn + 2NaOH → Na? ZnO? + H?
ii. 4Au + 8NaCN + O? + 2H? O → 4Na [Au (CN)? ]+ 4NaOH
iii. Cu + 4HNO? → Cu (NO? )? + 2NO? + 2H? O
(conc.)
Register to get relevant
Questions & Discussions on your feed
Ask & Answer
Panel of Experts