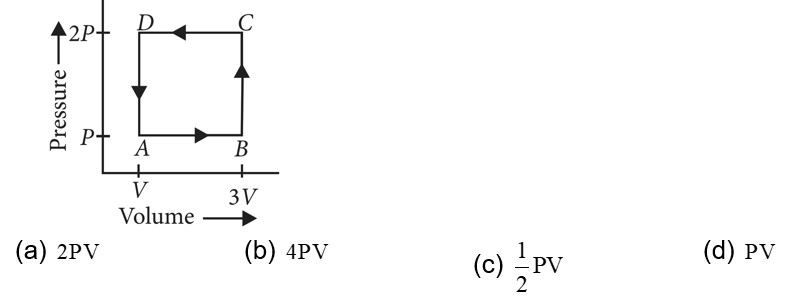Ask & Answer: India's Largest Education Community
All Questions
New Question
4 months agoContributor-Level 10
NH3 can be liquefied most easily due to presence of strong intermolecular H-bonding among NH3 molecules.
New Question
4 months agoContributor-Level 10
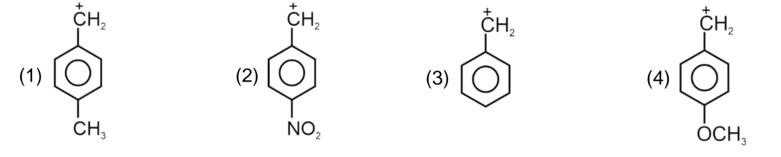
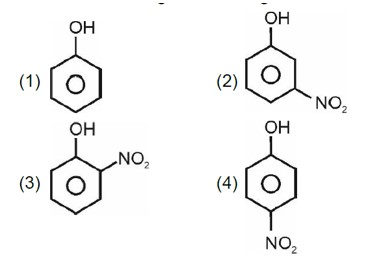
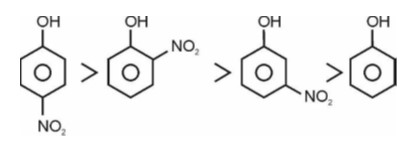
Electron withdrawing group decreases the stability of carbocation.
Correct order of stability of carbocation is
New Question
4 months agoContributor-Level 7
Candidates will be alloted the VTUEEE exam centres based on the preferences filled by them in the application form. While filling the application form, there will be options to select our preferred exam centre. That is when candidates can choose the centre of their choice.
New Question
4 months agoContributor-Level 10
New Question
4 months agoContributor-Level 10
t1/2 = [A]1-n
for second order (n = 2) reaction : t1/2 is proportional to 1/ [A ]
New Question
4 months agoContributor-Level 10
To apply to Stony Brook University, international applicants are required to follow the steps given below:
- Choose a program from more than 200 academic options, and review the requirements for their eligibility.
- UG students must register on the SUNY App or Common App, whie PG students are required to register on the University portal. Before the Stony Brook admissions deadline, applicants must submit a variety of documents, including official transcripts, letters of recommendation, essays, standardized test scores (optional), English language proficiency, and more.
- Pay the application fee worth USD 50 (INR 4.4 K) for UG programs and USD 1
New Question
4 months agoContributor-Level 10
The last date for the SHIATS Exam has been already passed, the last date was 10th July 2025 and its counselling session began on 22nd July 2025.
New Question
4 months agoContributor-Level 10
More the number of ions in solution, more will be the specific conductance
Ionic conductivity of H+ is more than that of Na+
New Question
4 months agoContributor-Level 9
In a cyclic process,
In a cyclic process work done is equal to the area under the cycle and is positive if the cycle is clockwise and negative if anticlockwise
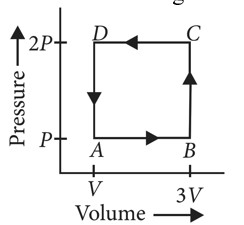
According to first law of thermodynamics
or
i.e., heat supplied to the system is equal to the work done
So heat absorbed,
Heat rejected by the gas
New Question
4 months agoContributor-Level 10
The primary reason for this is that silver is a quite expensive material to be used on such a large scale. Also, it doesn't have a much longer lifespan and tarnishes easily. So copper is used for electrical wiring because it offers low resistance to electric current is comparatively much more safer option to be commercially used.
New Question
4 months agoContributor-Level 10
Li+ has the largest size in aqueous solution due to its high polarizing power.
New Question
4 months agoNew Question
4 months agoContributor-Level 7
The VTUEEE exam centres are the designated venues where the VTUEEE exam will be held.
Register to get relevant
Questions & Discussions on your feed
Ask & Answer
Panel of Experts