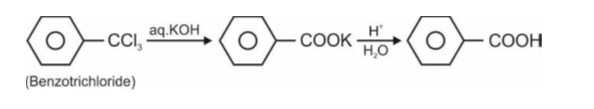
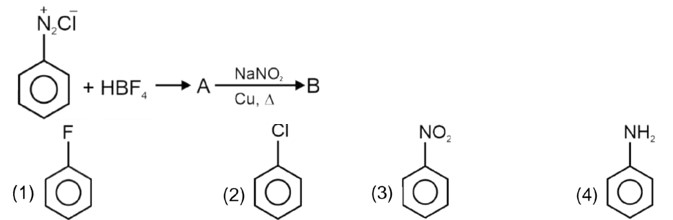
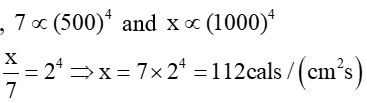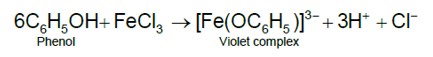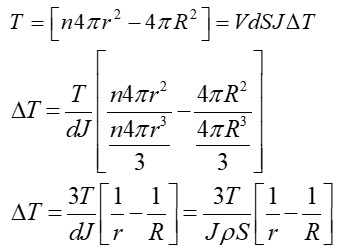Ask & Answer: India's Largest Education Community
All Questions
New Question
4 months agoContributor-Level 9
Rate of heat radiated at (227 +
Let rate of heat radiated at (727+ 273) K = xcals/ (cm2s)
By Stefan's law
New Question
4 months agoContributor-Level 10
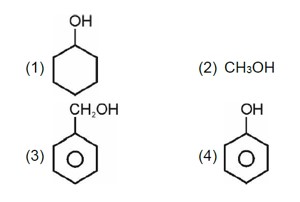
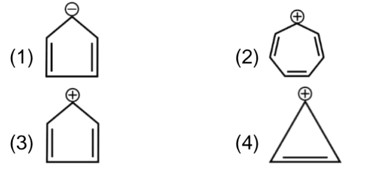
In (Ph)2C = CHCN3, no spatial variation is possible
It will not show geometrical isomerism.
New Question
4 months agoContributor-Level 10
The MBA programme at SBU is accredited by the AACSB, and students can choose from a range of streams which includes: Management, Finance, Marketing, and Entrepreneurship.
The course aims to give students a clear understanding of business principles, along with hands-on learning.
The course is taught by skilled business experts, frequently using real-world case studies and projects to improve the quality of learning.
New Question
4 months agoContributor-Level 10
Alkene on reaction with cold, dilute aqueous solution of KMnO4 produces vicinal glycols.
New Question
4 months agoNew Question
4 months agoContributor-Level 10
Admissions to London College of Music's UG courses have been started for upcoming academic session.
Interested undergraduate students can submit their respective applications via the UCAS portal by Jan 29, 2026.
New Question
4 months agoContributor-Level 10
The maximum prescribed concentration of manganese in drinking water is 0.05 ppm.
New Question
4 months agoContributor-Level 10
Correct order of increasing field strength of ligands is : I– < OH– < NCS–
New Question
4 months agoContributor-Level 10
EAN = Atomic No. – Oxidation state + 2 * CN
= 26 – 2 + 2 * 6
= 36.
New Question
4 months agoBeginner-Level 5
The admission procedure for Journalism and Mass Media at Chandigarh University is simple and transparent. Prospective students must fulfill eligibility requirements, generally completing 10+2 from a recognised board. Applications can be submitted online or offline, followed by document verification. Depending on the program, candidates may be required to attend counseling sessions or interviews, which help assess aptitude and interest in media studies. The university also provides information about course structure, practical labs, industry internships, and scholarship opportunities during the admission process. Once selected, students
Register to get relevant
Questions & Discussions on your feed
Ask & Answer
Panel of Experts