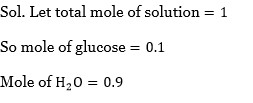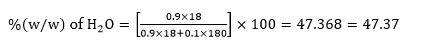A 0.5 percent solution of potassium choride was found to freeze at -0.24°C. The percentage dissociation of potassium chloride is__________. (Nearest integer)
(Molal depression constant for water is 1.80 K kg mol-1 and molar mass of KCl is 74.6 g mol-1)
A 0.5 percent solution of potassium choride was found to freeze at -0.24°C. The percentage dissociation of potassium chloride is__________. (Nearest integer)
(Molal depression constant for water is 1.80 K kg mol-1 and molar mass of KCl is 74.6 g mol-1)
0.5 % KCl solution has molality (m) =
1 - a a a
And I =
1.976 = 1 + a
% = 97.6%
the nearest 98.
Similar Questions for you
Kf = 1.86
Using, density of water = 1g / mL
i = ?
i = 1.344
Now, using
n for ClCH2COOH = 2
a =
Using
&nb
Kindly consider the following Image
0.93 = 1.86 × 1 × i
i =
For acetone solution,
For Benzene solution,
=
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry Solutions 2025
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering