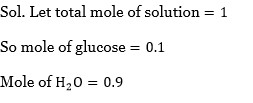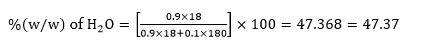The mole fraction of glucose (C?H??O?) in an aqueous binary solution is 0.1. The mass percentage of water in it, to the nearest integer, is
The mole fraction of glucose (C?H??O?) in an aqueous binary solution is 0.1. The mass percentage of water in it, to the nearest integer, is
3 Views|Posted 5 months ago
Asked by Shiksha User
1 Answer
Similar Questions for you
0.5 % KCl solution has molality (m) =
1 - a a a
And I =
1.976 = 1 + a
% = 97.6%
the nearest 98.
Kf = 1.86
Using, density of water = 1g / mL
i = ?
i = 1.344
Now, using
n for ClCH2COOH = 2
a =
Using
&nb
0.93 = 1.86 × 1 × i
i =
For acetone solution,
For Benzene solution,
=
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
66K
Colleges
|
1.2K
Exams
|
6.9L
Reviews
|
1.8M
Answers
Didn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
or
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
or
See what others like you are asking & answering