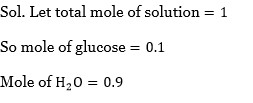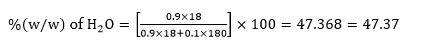A radioactive element has a half life of 200 days. The percentage of original activity remaining after 83 days is___________. (Nearest integer)
(Given : antilog 0.125 = 1.333, antilog 0.693 = 4.93)
A radioactive element has a half life of 200 days. The percentage of original activity remaining after 83 days is___________. (Nearest integer)
(Given : antilog 0.125 = 1.333, antilog 0.693 = 4.93)
83 =
0.125 =
= 1.333
Activating remaining =
= 75%
Similar Questions for you
0.5 % KCl solution has molality (m) =
1 - a a a
And I =
1.976 = 1 + a
% = 97.6%
the nearest 98.
Kf = 1.86
Using, density of water = 1g / mL
i = ?
i = 1.344
Now, using
n for ClCH2COOH = 2
a =
Using
&nb
Kindly consider the following Image
0.93 = 1.86 × 1 × i
i =
For acetone solution,
For Benzene solution,
=
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Didn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering